Patent Term Extension: What It Means for Drug Access and Prices
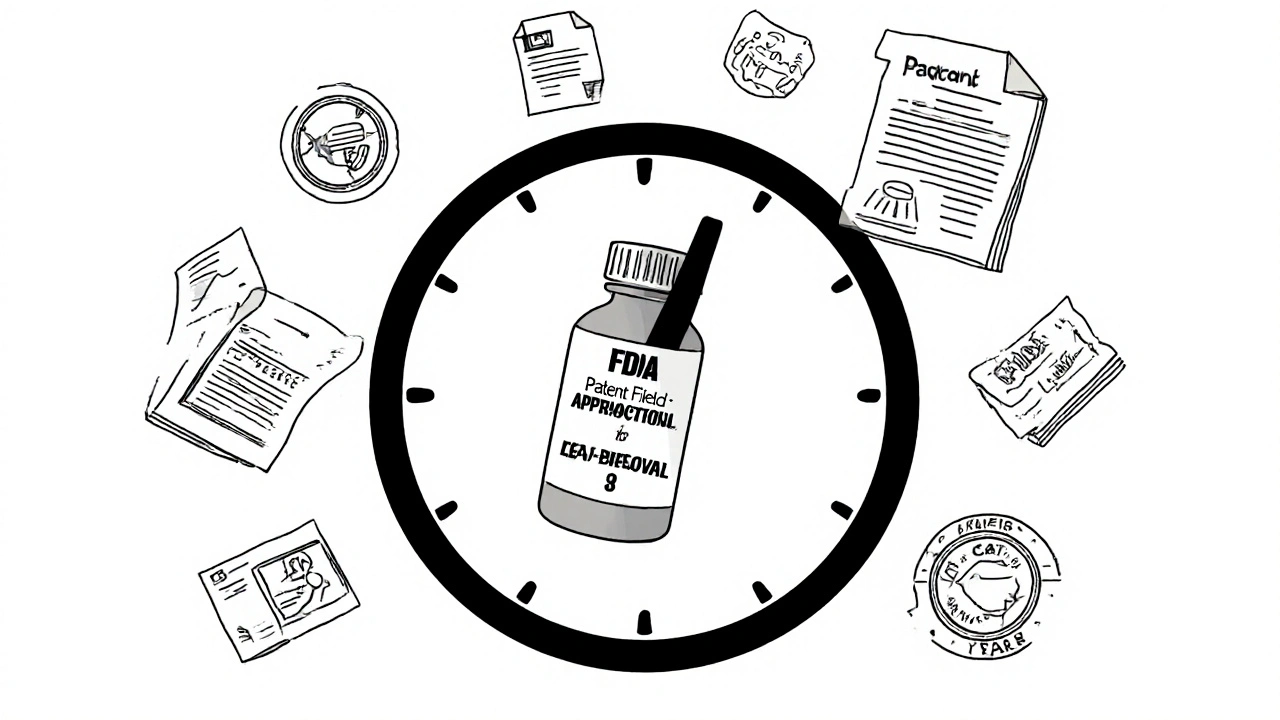
When a new drug hits the market, its patent term extension, a legal adjustment that adds time to a drug’s patent after FDA approval to compensate for review delays. Also known as drug patent restoration, it’s a tool built into U.S. law to balance innovation and access. Without it, companies might lose most of their exclusive selling time just waiting for the FDA to approve their drug—sometimes more than half of the original 20-year patent. That’s why Congress passed the Hatch-Waxman Act in 1984: to give drug makers a fair shot at recouping R&D costs without blocking generics forever.
But FDA exclusivity, a separate period of market protection granted by the FDA even before the patent expires often runs alongside patent term extension. For example, a new chemical entity might get five years of exclusivity, while its patent gets extended by three years. These two layers can delay generic versions for over a decade, even if the original patent would’ve expired sooner. This isn’t just legal jargon—it directly affects how soon you can buy a cheaper version of your medication. Meanwhile, generic drugs, lower-cost copies of brand-name medicines that enter the market after patent and exclusivity periods end are the main reason drug prices drop. But if patent term extension is used aggressively, those generics stay locked out longer than intended.
What you’ll find in the posts below isn’t a dry legal breakdown—it’s real-world insight into how this system plays out. You’ll see how patent term extension affects access to asthma meds during pregnancy, why some diabetes drugs cost more for years after approval, and how the same rules that protect new treatments can also delay life-saving generics. These aren’t abstract policies—they show up in your prescription bottle, your pharmacy bill, and your doctor’s recommendations. Whether you’re managing a chronic condition, helping an aging parent, or just trying to understand why some drugs are so expensive, this collection gives you the facts without the fluff.
When Do Drug Patents Expire? Understanding the 20-Year Term and Real-World Timelines
Drug patents are legally 20 years long, but most expire in 7-12 years due to R&D delays. Learn how patent extensions, regulatory exclusivity, and legal strategies affect when generics become available.
read more