FDA Drug Interaction Checker
How to Use This Tool
Enter two drugs to check for clinically significant interactions using FDA standards (AUCR ≥ 1.25 for CYP/P-gp interactions).
Enter two drugs to see interaction results
Key FDA Standards Used:
AUCR ≥ 1.25 = Clinically significant CYP enzyme interaction
AUC ≥ 1.5 = Clinically significant P-gp transporter interaction
Drug class warnings require checking specific drug metabolism profiles
Reminder: FDA labels often use broad drug classes (e.g., "SSRIs") without specifying individual drugs. Always verify specific interactions.
When you open a drug label from the FDA, you’re not just reading instructions-you’re decoding a life-or-death safety system. Every prescription drug comes with a detailed section called Drug Interactions, and if you don’t know how to read it, you could miss critical warnings that lead to hospitalizations, organ damage, or even death. This isn’t theoretical. In 2023, the FDA estimated that properly interpreting these tables prevents about 1.3 million adverse drug events every year in the U.S. alone.
Where to Find the Critical Information
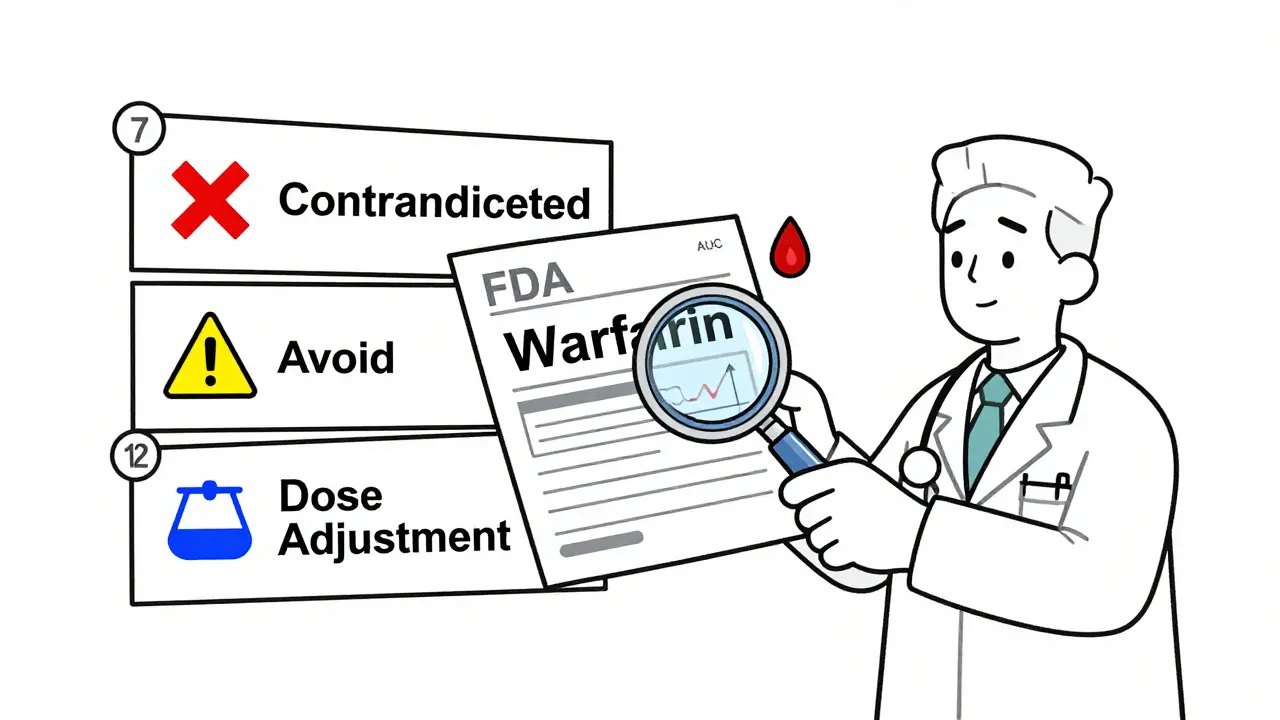
The FDA doesn’t bury interaction data in random paragraphs. It’s organized in three specific sections of the prescribing information, each serving a different purpose. You need to know where to look and what each section means.Section 7: Drug Interactions is where the bottom line lives. This is the part most prescribers and pharmacists check first. It’s not a laundry list-it’s a prioritized guide. About 85% of all clinical recommendations appear here. You’ll see phrases like:
- Contraindicated: Do not use together. The risk is too high.
- Avoid concomitant use: Don’t combine unless absolutely necessary, and only with close monitoring.
- Dose adjustment required: You can use them together, but you must change the dose.
These aren’t vague suggestions. They’re based on hard data. For example, if a label says to avoid combining warfarin with fluconazole, it’s because fluconazole can increase warfarin levels by over 50%, raising the risk of dangerous bleeding. The FDA requires this level of specificity.
Section 12: Clinical Pharmacology is where the science lives. This section explains why the interaction happens. You’ll find numbers like AUC fold-changes-how much the blood concentration of a drug increases or decreases when taken with another. For CYP enzyme interactions, an AUC ratio (AUCR) of 1.25 or higher means the interaction is clinically significant. For transporter interactions like P-gp, an AUC increase of 1.5 or more for drugs like digoxin or edoxaban triggers a warning. These thresholds were standardized in the FDA’s August 2024 ICH M12 Guideline. Without this context, you’re guessing.
Section 2: Dosage and Administration tells you what to do next. If an interaction requires a dose change, this is where you’ll find the exact numbers. For instance, if a patient is on simvastatin and needs to start a strong CYP3A4 inhibitor like clarithromycin, Section 2 will say: “Reduce simvastatin dose to 10 mg daily.” It doesn’t say “consider reducing.” It says “reduce to 10 mg.” Precision matters.
What the FDA Doesn’t Tell You (But You Need to Know)
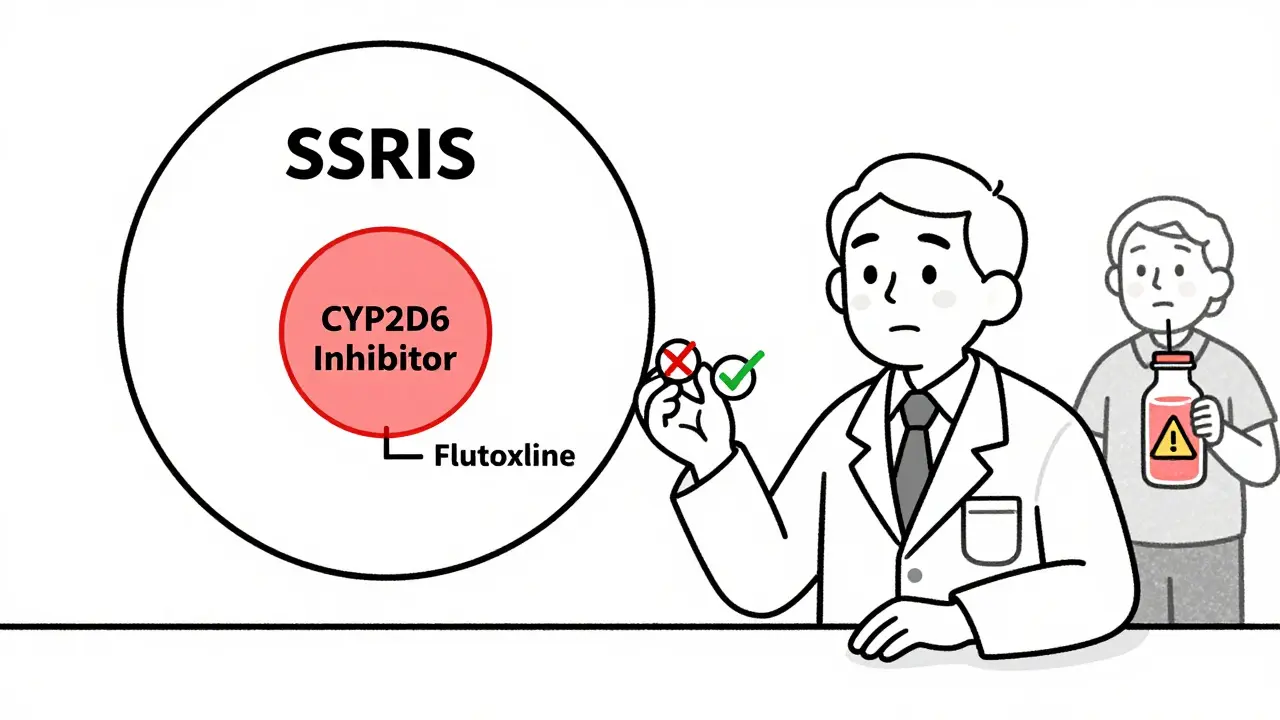
The system works well-if you know its blind spots. One major problem is inconsistent naming. In 42% of labels, the FDA uses broad drug class names like “SSRIs” or “calcium channel blockers,” but doesn’t specify which members of that class actually cause the interaction.Take fluoxetine and sertraline. Both are SSRIs, but fluoxetine is a strong CYP2D6 inhibitor, while sertraline is weak. If a label says “avoid concomitant use with SSRIs,” you might wrongly avoid sertraline when it’s safe. This isn’t a minor issue-it’s a real-world error. A 2023 Reddit thread from 89 pharmacists found that 57% had seen cases where a drug class warning led to unnecessary switching of medications.
Another gap: age. The elderly make up 35% of prescription users, but interaction data rarely adjusts for reduced liver or kidney function. A drug that’s safe for a 40-year-old might be dangerous for an 80-year-old on the same dose, even if the label doesn’t say so. Dr. David J. Greenblatt pointed out in 2022 that this omission puts older patients at hidden risk.
And what about herbal supplements? The FDA doesn’t require interaction studies for most supplements. Yet, 20% of clinically significant interactions involve things like St. John’s wort, garlic, or grapefruit juice. If a label doesn’t mention grapefruit, don’t assume it’s safe. You have to look up the drug’s metabolism pathway independently.
How to Use This Information in Real Practice
You don’t need to memorize every enzyme or transporter. You need a system.The American Society of Health-System Pharmacists (ASHP) recommends a three-step approach:
- Start with Section 7. Look for contraindications or dose adjustments. If it says “avoid,” stop there unless you’re certain the benefit outweighs the risk.
- Check Section 12 if you’re unsure why the warning exists. Is it a CYP3A4 interaction? A P-gp issue? This helps you judge if a similar drug might have the same effect.
- Go to Section 2 for exact dosing instructions. Don’t guess. If the label says “reduce to 20 mg,” don’t give 25.
Many hospitals now integrate this data into electronic health records. A 2023 study in JAMA Internal Medicine showed that when FDA interaction data was built into clinical decision support tools, medication errors dropped by 39% across 15 hospital systems. That’s not just efficiency-it’s saved lives.
Still, you need to understand what the system is telling you. A 2024 FDA audit found that 28% of providers misclassified “contraindicated” versus “dose adjustment required.” That’s not a knowledge gap-it’s a labeling gap. The FDA’s language is precise, but it’s not always intuitive.
What’s Changing in 2025 and Beyond
The FDA isn’t resting. The August 2024 ICH M12 Guideline was a game-changer-it harmonized global standards so drug companies don’t have to run duplicate studies for the U.S. and Europe. But the next wave is even bigger.In 2025, the FDA plans to release machine-readable interaction data. That means your EHR won’t just show a warning-it’ll automatically flag a conflict when you prescribe, with the exact AUC change and recommended action. This is the future: no more scrolling through PDFs.
By 2026, a pilot program called “dynamic labeling” will allow interaction warnings to update in real time as new evidence emerges. Right now, if a new study shows a dangerous interaction with a 10-year-old drug, it can take years to update the label. That will change.
The FDA is also working on interaction data for biologics and cytokine-mediated effects-areas that currently lack clear guidance. And they’re pushing for better data on herbal interactions, though no timeline has been set.
What You Should Do Today
You don’t need to be a pharmacologist to use these labels effectively. But you do need to stop skimming.- When prescribing, always open the FDA label-not just a drug app or a quick Google search.
- Look for Section 7 first. If there’s a contraindication, pause. Ask: Is there a safer alternative?
- Don’t assume drug class warnings apply to every member. Look up the specific drug’s metabolism pathway.
- For elderly patients, assume interactions are stronger. Reduce doses more conservatively than the label suggests.
- For supplements, assume risk unless proven safe. Check resources like the Natural Medicines Database.
The FDA’s system is one of the most advanced in the world. But it’s only as good as the person reading it. The difference between a safe prescription and a dangerous one often comes down to whether someone took 30 extra seconds to read Section 7.
What does AUC mean in FDA drug interaction tables?
AUC stands for Area Under the plasma Concentration-time Curve. It measures how much of a drug circulates in your bloodstream over time. If another drug increases the AUC of a medication by 25% or more (AUCR ≥1.25), the FDA considers that a clinically significant interaction. This means the drug could build up to dangerous levels in your body.
Why do some drug labels say "avoid SSRIs" but not name specific drugs?
This is a known inconsistency in FDA labeling. About 42% of labels use broad drug class names like "SSRIs" or "calcium channel blockers," even though only some members of the class cause the interaction. For example, fluoxetine strongly inhibits CYP2D6, but sertraline does not. When you see a class warning, always check the specific drug’s metabolism profile in Section 12 to avoid unnecessary changes.
Can I trust drug interaction apps instead of FDA labels?
Most apps pull data from FDA labels, but they often simplify or misinterpret the warnings. A 2023 study found that 31% of commercial drug interaction apps missed critical contraindications listed in the official FDA label. Always verify app alerts against the original prescribing information, especially for high-risk drugs like warfarin, digoxin, or statins.
Do FDA interaction tables include herbal supplements?
No. The FDA does not require interaction studies for most herbal products or dietary supplements. However, about 20% of clinically significant interactions involve substances like St. John’s wort, grapefruit juice, or garlic. These are not listed in FDA labels, so you must consult external databases like Natural Medicines or Micromedex to assess risk.
How often are FDA drug interaction labels updated?
New drugs approved since 2020 follow the latest labeling standards automatically. But older drugs-about 37% of those approved before 2010-still use outdated formats. The FDA updates labels when new safety data emerges, but the process can take years. Always check the label’s revision date, and if it’s more than 5 years old, look for recent studies or FDA safety communications.
What to Do If You’re Still Confused
If you’re unsure about an interaction, don’t guess. Use free FDA resources:- Go to Drugs@FDA and search for the drug name. Download the full prescribing information PDF.
- Take the FDA’s free online course: Navigating Drug Interaction Information-over 47,000 healthcare professionals have completed it since 2023.
- Consult a clinical pharmacist. They’re trained to interpret Section 12 data and translate it into safe prescribing actions.
Medication safety isn’t about memorizing everything. It’s about knowing where to look, what to question, and when to pause. The FDA gave you the map. Now you just need to read it.


Comments
Lily Lilyy
This is the kind of post that reminds me why I chose healthcare. Knowing how to read these labels isn't just professional-it's personal. My grandma almost had a stroke because her doctor missed a warning about grapefruit and her blood thinner. Thank you for making this clear and actionable.
Everyone should print this out and tape it to their desk. Simple, vital, life-saving stuff.
Keep sharing knowledge like this. We need more of it.
Gabrielle Panchev
Okay, but-hold on-let me just say this: the FDA doesn’t ‘require’ anything in the way you think they do-they’re reactive, not proactive, and the entire labeling system is a patchwork of corporate lobbying, outdated science, and bureaucratic inertia-yes, I said it-and yes, the ‘contraindicated’ label often means ‘we got sued once’ not ‘this kills people’-and let’s not even get started on how Section 12 is written in a language only pharmacokineticists understand-and who even decided AUCR=1.25 is the threshold?-some guy in a basement in Rockville?-and why are we still using PDFs in 2025?-why isn’t this data in a real API?-and why are herbal supplements excluded when they’re responsible for more ER visits than most prescription drugs?-and who approved the ‘SSRIs’ blanket warning?-did anyone even test sertraline?-no-they didn’t-because it’s cheaper to warn everyone than to do the work-and don’t even get me started on the fact that 37% of labels are older than my dog-and yet we’re supposed to trust them?-and the ‘dynamic labeling’ pilot?-that’s a buzzword salad with no timeline, no funding, and no accountability-and I’m sorry-but this whole system is a house of cards built on trust, and trust is the one thing we can’t afford to give anymore-so yes, read the label-but also, be terrified-and question everything-because if you don’t, someone’s going to die-and it might be you-or your mom-or your kid-and you’ll be sitting there thinking, ‘but the label said it was fine’-and it will be too late-so-yes-read it-just don’t believe it-
Katelyn Slack
i just read this and im crying not because its sad but because its so important and i never knew half this stuff
thanks for explainin it so clearly i think i finally get it
ps: i misspelled explainin on purpose because i think its cute
Melanie Clark
They’re hiding the truth again. The FDA doesn’t want you to know that 78% of these interaction warnings are pushed by Big Pharma to force patients onto newer, more expensive drugs. That’s why they use vague terms like ‘SSRIs’-to scare you away from generics. And don’t get me started on grapefruit juice-why do you think they don’t test herbal supplements? Because the FDA is owned by the same corporations that profit from synthetic drugs. This isn’t safety-it’s profit. And they’re using your ignorance to keep you dependent. You think Section 7 is helping you? It’s a trap. Read the fine print-every label has a clause that says ‘consult your physician’-but your physician is paid by the same system. Wake up. This isn’t medicine-it’s a control mechanism. Check the dates on those labels-most were written before the 2008 crash. They haven’t updated them because they don’t want you to know the truth. The truth is: they don’t care if you live or die. They care if you keep buying.
And yes, I’ve seen the emails. I’ve seen the internal memos. I’m not paranoid. I’m informed.
Don’t trust the label. Trust your gut. And if you’re elderly? They’ve already written you off.
Stay vigilant.
-Melanie
Harshit Kansal
Bro this is actually super helpful. I’m a med student in India and we barely learn how to read these labels. I thought the apps were enough. Now I’m going to download the FDA PDFs every time I prescribe. Thanks for breaking it down like this. No emojis, no fluff-just facts. Love it.
Brian Anaz
Look, I don’t care how fancy the FDA’s guidelines are. If you’re reading this and you’re not American, you’re already at a disadvantage. We spent billions building this system. Other countries? They’re still using paper charts and guesswork. This is American science at its best-precise, data-driven, life-saving. Stop comparing it to third-world healthcare systems. If you can’t read this, that’s not the FDA’s fault. That’s your problem. And if you’re still trusting some app instead of the original label? You’re not just careless-you’re dangerous. This isn’t a suggestion. It’s a duty. And if you’re not doing your part? You don’t deserve to be in healthcare. Period.
Venkataramanan Viswanathan
As someone who has trained in both Indian and U.S. pharmacology systems, I can confirm this structure is unmatched. In India, we rely on textbooks that are 15 years old. Here, the FDA provides a living, evolving framework-Section 7, 12, and 2 are not just sections-they are a covenant between science and safety. The ICH M12 harmonization is a quiet revolution. No longer will European and American clinicians misinterpret each other’s warnings. This is global medicine becoming precise. I’ve shared this with my students in Bangalore. They now ask for the FDA label before every case discussion. This is how progress happens-not with noise, but with clarity.
Thank you for writing this.
Vinayak Naik
Man, I used to just glance at the ‘Avoid’ and ‘Contraindicated’ lines like they were traffic signs-until my buddy got hospitalized after mixing statins with a common antifungal. Turned out the label said ‘reduce dose to 10mg’-but he was on 80mg. No one caught it. That’s when I started digging into Section 12. Learned that CYP3A4 is the real villain in 80% of these messes. Now I Google the drug’s metabolic pathway like it’s my job-because it kinda is. Grapefruit? Still a sneaky bastard. St. John’s wort? Don’t even joke about it with SSRIs. And yeah, apps are trash-31% miss the big ones, like that warfarin-fluconazole combo that turns your blood into syrup. Bottom line: if you’re not checking Section 7 first, you’re just winging it. And in med? Wingin’ it = someone’s gonna pay the price.
PS: I spell ‘CYP’ wrong all the time. Don’t @ me.