Problematic Generics: What Goes Wrong and How to Stay Safe
When you pick up a problematic generics, generic medications that don’t perform as expected due to formulation issues, inconsistent manufacturing, or poor bioequivalence testing. Also known as substandard generics, these drugs look identical to brand-name pills but may not deliver the same results—or could even cause harm. It’s not about cost. It’s about quality control. The FDA approves most generics as safe and effective, but a small number slip through the cracks. These are the ones that cause unexpected side effects, fail to control blood pressure, or don’t stop seizures. And they’re not always easy to spot.
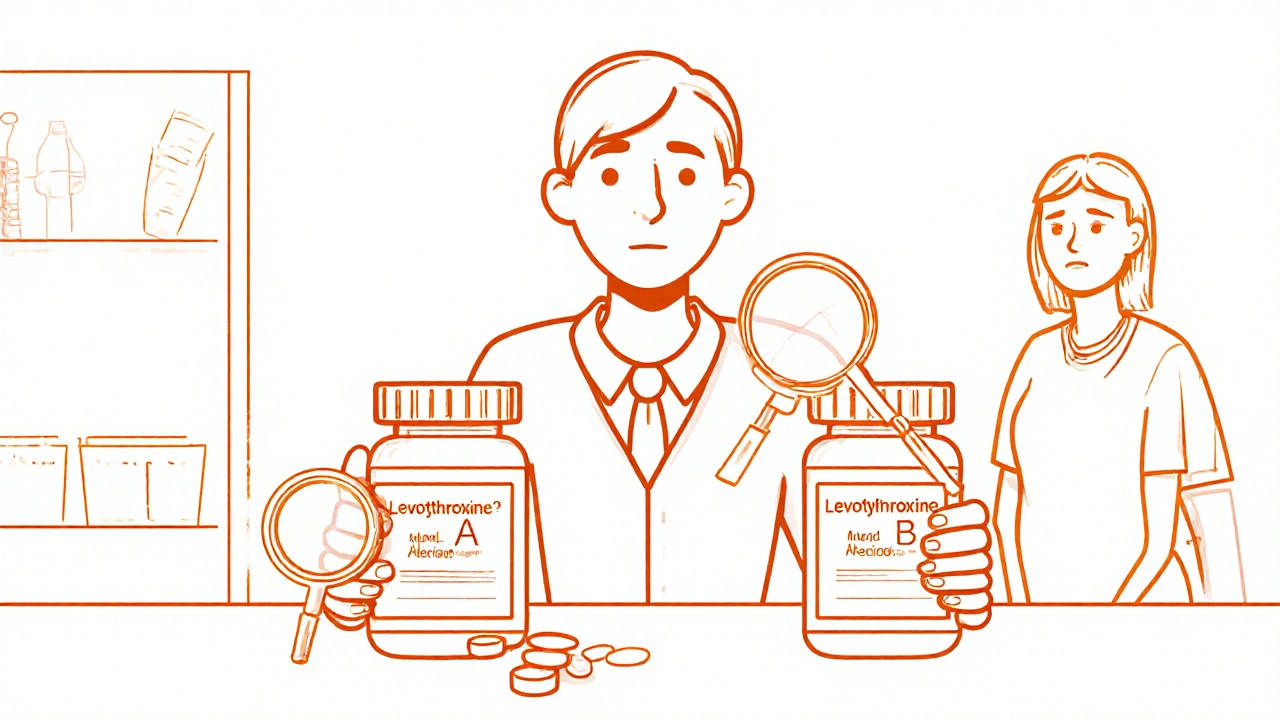
One major reason problematic generics exist is because of how bioequivalence, the process that proves a generic drug releases the same amount of active ingredient into the body as the brand version is tested. The tests are done on healthy volunteers under ideal conditions. But real people? They have different stomachs, different metabolisms, and different health conditions. A generic that works fine for a 25-year-old might not work at all for a 70-year-old with kidney issues. And when manufacturers cut corners on inactive ingredients—like fillers or coatings—it can change how fast the drug dissolves. That’s why some people report their generic blood pressure pill suddenly stops working, or their seizure frequency jumps. It’s not in their head. It’s in the pill.
Another issue? medication errors, mistakes made when switching between brands and generics, or when pharmacies substitute without warning. Doctors don’t always know which generic their patient got. Pharmacies often switch brands based on price, not performance. And patients? They assume the blue pill is the same as the red one. But if the inactive ingredients change, or the dissolution rate shifts, it can make a real difference. We’ve seen cases where people on generic levothyroxine suddenly needed dose adjustments after a switch—not because their thyroid changed, but because the new version absorbed differently. The same thing happens with epilepsy drugs, antidepressants, and even blood thinners.
It’s not all bad news. Most generics are fine. But the ones that aren’t? They’re the ones you hear about in whispers: the patient who had a stroke after switching, the mom whose asthma got worse, the veteran whose PTSD meds stopped working overnight. These aren’t rare. They’re just underreported. The system assumes generics are interchangeable. But biology doesn’t care about labels. It cares about what’s inside—and how it behaves in your body.
What you’ll find here are real stories, real data, and real advice from people who’ve been through it. We’ll show you how to spot a problematic generic before it affects you, how to talk to your pharmacist about consistency, and when to push back and ask for a specific brand. You’ll learn what to look for on the label, how to track changes in how you feel after a switch, and what to do if your meds suddenly stop working. This isn’t about fear. It’s about control. You deserve a pill that works—every time.
When Pharmacists Must Flag Problematic Generic Medications
Pharmacists play a vital role in identifying problematic generic medications that may cause therapeutic failure or adverse effects. Learn when and how to flag issues with NTI drugs, look-alike names, and inconsistent formulations.
read more